Our Pipeline
Creating a new standard of cNIBP.
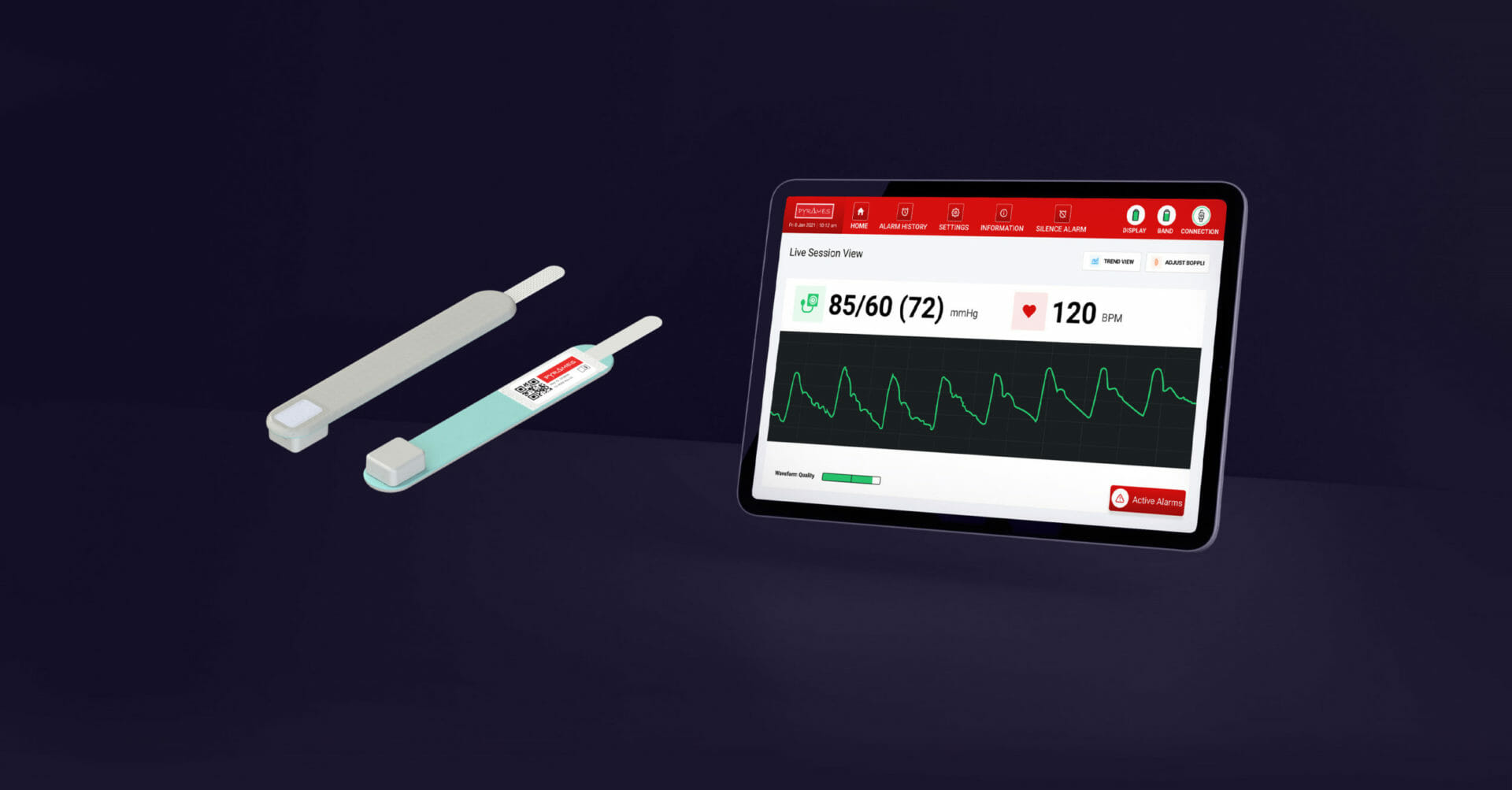
PyrAmes has developed a unique sensor platform that provides continuous blood pressure monitoring in a lightweight, comfortable, wireless package. With its paper-thin, flexible sensor and compact wearable form factor, it is an alternative to arterial lines and inflatable cuff-based blood pressure monitoring systems.
PyrAmes is proud to announce that our platform has been granted Breakthrough Device Designation (BDD) and 510(k) market clearance from the FDA for Boppli®, our novel continuous, non-invasive blood pressure (cNIBP) monitoring system intended for use in neonatal critical care settings.
Our Product Pipeline

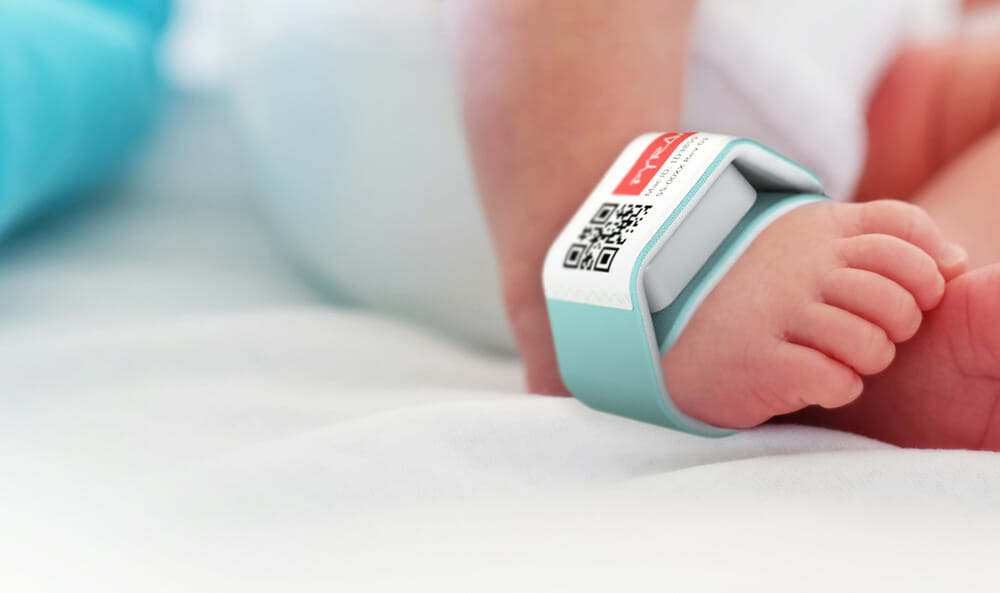
Pediatric Inpatient
Boppli®
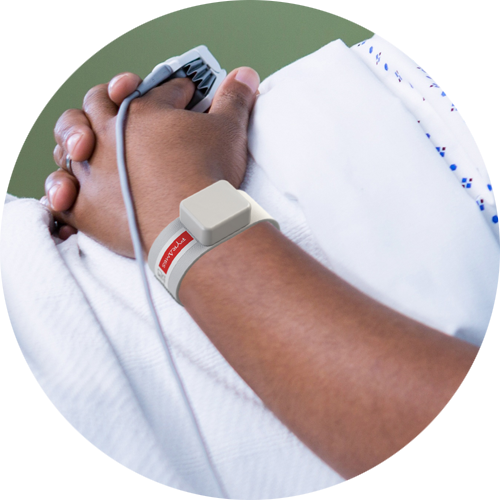
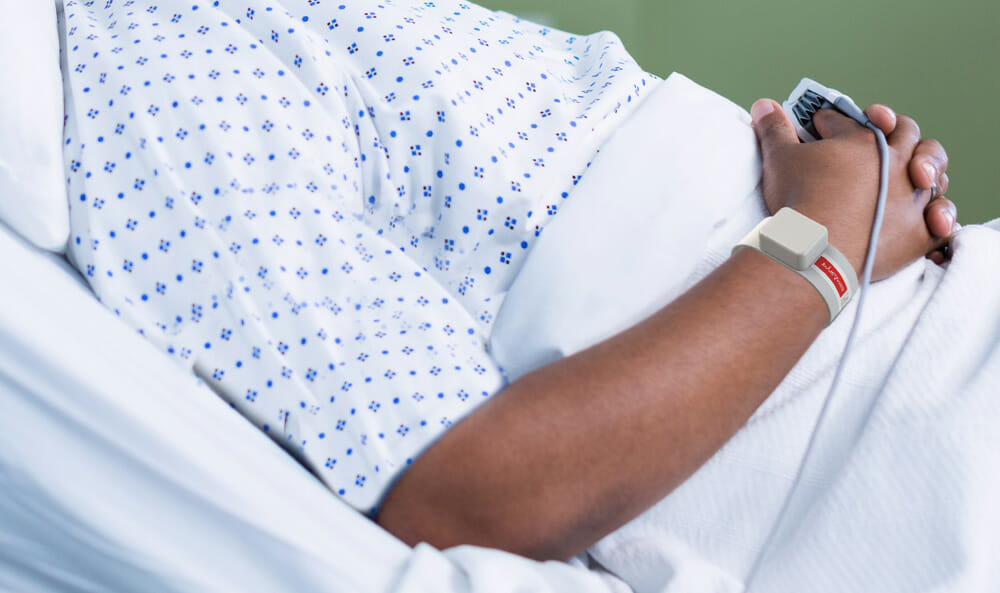
Adult Inpatient
Bosimi™

Adult Outpatient
Bosimi@Home™ Monitoring
Our Advanced Sensor
PyrAmes’ proprietary sensor technology has the potential to reshape how blood pressure conditions are diagnosed and managed.
A cNIBP sensor can help a clinical team provide timely intervention during a hypotensive crisis for a patient in critical or emergency care situations, without the pain or risk of an arterial line. This has the potential to directly cause significant reductions in strokes, heart attacks and other serious conditions related to hypertension.
![$image['alt'];](https://pyrameshealth.com/wp-content/uploads/2021/04/pipeline-boppli-device.png)